Perform conceptualization & detail process design for upstream & downstream Bio-pharma. Design CIP & SIP procedures. Riboflavin testing of all the associated systems, parameter calculations, instrument calibration, review of device sequencing & drafting URS & FRS according to cGMP.
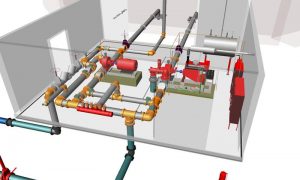
Perform process calculations & simulation using fluid dynamics simulation software (e.g. AFT Fathom). Assist in skid design, supervise Installation, Start-Up, Debug, Commissioning & troubleshooting process skids. Perform FAT, SAT & Validation. Develop P&IDs. Design mechanical equipment. Provide skid layout assistance. Develop & maintain ETOP. Walk down Production Bio-Reactors, Media & Buffer systems, fermentation & purification skids. Hazard assessment, RCA & safety validation per OSHA requirements. Design of HVAC, Chiller system, required utilities, BMS for AHU.
Requirements: Master’s degree or equivalent in Industrial or Mechanical Engineering or
related field + 1 year experience in a position involving similar duties/technical capabilities.

































Recent Comments