If you are involved in any type of manufacturing that is regulated by the FDA, FDA regulatory consulting firms can help you!
FDA consultants (who are all former employees of the FDA or have extensive industry experience) can assist with all phases of the manufacturing process, from single rooms to entire plants, computer systems to manufacturing and processing equipment, design to verifications and validations.
FDA consulting and FDA training services typically include:
- master and batch record design and reviews
- specification development (components, in-process, and finished product)
- supplier audits
- medical device design history file (21 CFR 820.30)
- dietary supplements product design
- equipment verification
- process validation (prospective validation, retrospective validation, and revalidation)
- DQ (Design Qualification)
- IQ (Installation Qualification)
- OQ (Operational Qualification)
- PQ (Prospective Qualification)
- cleaning validation (all industries)
- GMP (Good Manufacturing Practices) and HACCP (Hazard Analysis and Critical Points)
- stability studies
- clean rooms
- sterility
- calibration
- PM (Preventive Maintenance) and DM (Demand Maintenance)
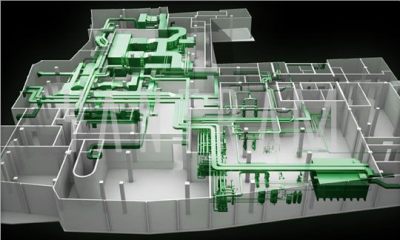
- plant design
- computer system development and validation
- ERP software (Enterprise Resource Planning)
- data collection and storage systems
- production equipment
- system software for manufactured products
FDA’s role in plant design:
- cGMP requirements
- Design & Construction features
- Lighting
- Ventilation, air filtration, air heating and cooling
- Plumbing
- Sewage & refuse
- Washing & Toilet facilities
- Sanitation
- Maintenance
- cGMP Coverage of design
FDA’s Compliance Programs provide instructions to FDA personnel for conducting activities to evaluate industry compliance with the Federal Food, Drug, and Cosmetic Act and other laws administered by FDA. Compliance Programs are made available to the public under the Freedom of Information Act.
Compliance Programs do not create or confer any rights for or on any person and do not operate to bind FDA or the public. An alternative approach may be used as long as the approach satisfies the requirements of the applicable statutes and regulations. FDA’s Compliance Programs are organized by the following program areas:- Biologics (CBER)
- Bioresearch Monitoring (BIMO)
- Devices/Radiological Health (CDRH)
- Drugs (CDER)
- Food and Cosmetics (CFSAN)
- Veterinary Medicine (CVM)
- Facilities & Equipment Systems
- Cleaning & maintenance
- Facility layout and air handling systems for prevention of cross-contamination (e.g. Penicillin, beta-lactams, steroids, hormones, cytotoxics, etc.)
- Specifically designed areas for the manufacturing operations performed by the firm to prevent contamination or mix-ups
- General air handling systems
- Control system for implementing changes in the building
- Lighting, potable water, washing and toilet facilities, sewage and refuse disposal
- Sanitation of the building, use of rodenticides, fungicides, insecticides, cleaning and
- Sanitizing agents
- Preapproval Coverage of Design/Preapproval Inspections/Investigations
The addition of any new drug to a production environment must be carefully evaluated as to its impact on other products already under production and changes that will be necessary to the building and facility. Construction of new walls, installation of new equipment, and other significant changes must be evaluated for their impact on the overall compliance with GMP requirements.
For example, new products, such as cephalosporins, would require that the firm demonstrate through appropriate separation and controls that cross-contamination cannot occur with regard to other products being made in the same facility. Also, facilities that may already be operating at full capacity may not have adequate space for additional products
Quality Systems Approach to Pharmaceutical cGMP Regulations
Quality by design means designing and developing a product and associated manufacturing processes that will be used during product development to ensure that the product consistently attains a predefined quality at the end of the manufacturing process.
Quality by design, in conjunction with a quality system, provides a sound framework for the transfer of product knowledge and process understanding from drug development to the commercial manufacturing processes and for post-development changes and optimization.
The CGMP regulations, when viewed in their entirety, incorporate the concept of quality by design. This guidance describes how these elements fit together.
For more information on FDA consulting services for plant design or to schedule a consultation, please contact us.











Recent Comments